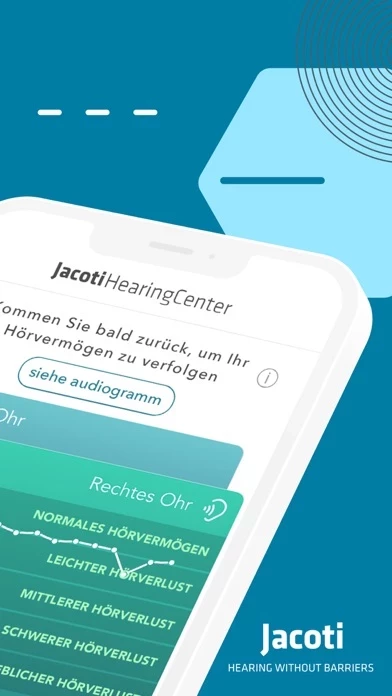
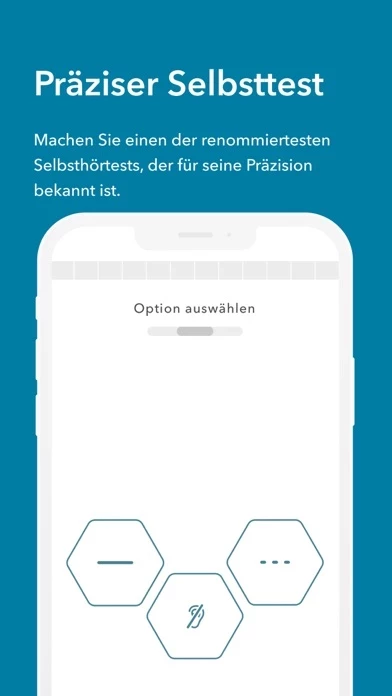
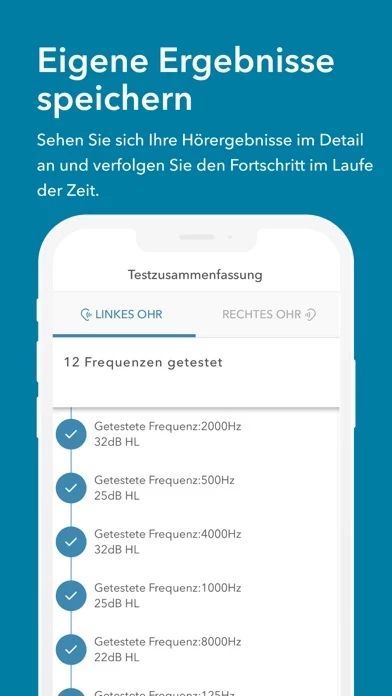
The intended use of Jacoti Hearing Center is for individuals to perform by means of acoustic signals with controlled intensity hearing self-evaluations in real-life environments without the assistance of a hearing expert.
When used by consumers in real-life environments without the assistance of a hearing expert, Jacoti Hearing Center is intended as a screening device for informational purposes.
In the United States of America, the intended use of Jacoti Hearing Center is limited to performing hearing assessments by means of acoustic signals with controlled intensity.
When used under the guidance of an audiologist or a hearing professional, Jacoti Hearing Center is intended to be used as a hearing fitting and hearing diagnostic audiometer.
Jacoti Hearing Center is a Class II FDA listed Medical Device, classified under product code EWO in the U.S and complies with the CE requirements for medical devices (CE 2797) in Europe.