It is listed under the registration number DE/CA71/21a/07/2.3/2017/0043. SaniQ Heart thus complies with the requirements of Council Directive 93/42/EEC on medical devices of the European Union.
You have the option to purchase a monthly (30 days) or yearly (365 days) subscription for creating premium reports, medication photos, individual weather and pollen flight data, Excel export, multi-device connectivity and improved medication management.
SaniQ Heart uses HealthKit to import previously captured data such as blood pressure, pulse, SpO2 and weight.
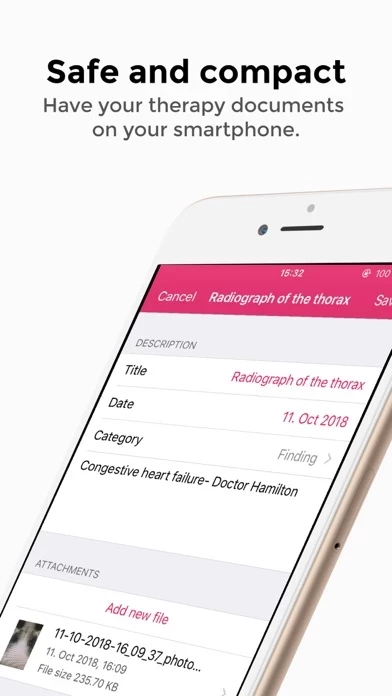
SaniQ Heart is a medical device developed, maintained and registered in Germany.
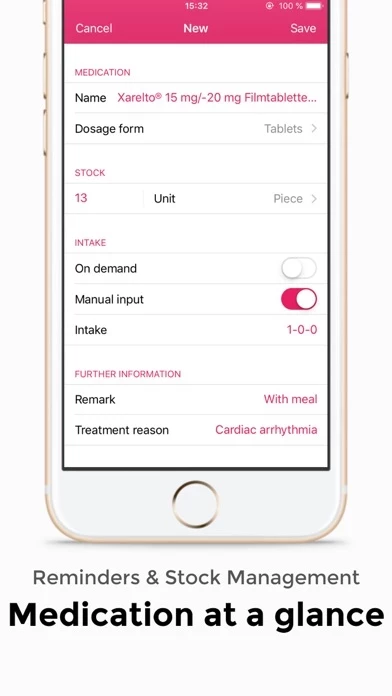
The SaniQ Heart app supports you with daily challenges: It reliably reminds you to take measurements and take medication.