It is registered under the number DE/CA71/21a/07/2.3/2017/0043. SaniQ Asthma thus fulfils the requirements of the 93/42/EEC Directive of the European Union Council on Medical Devices.
You have the option to purchase a monthly (30 days) or yearly (365 days) subscription for creating premium reports, medication photos, individual weather and pollen flight data, Excel export, multi-device connectivity and improved medication management.
SaniQ Asthma is a medical device developed, maintained and registered in Germany.
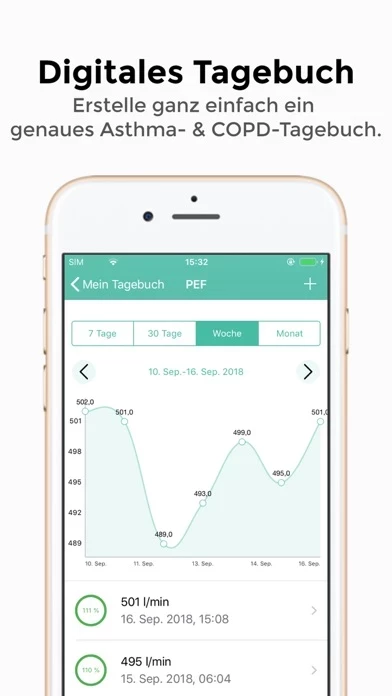
SaniQ Asthma uses HealthKit to import previously captured data such as peak flow, FEV1 and weight.
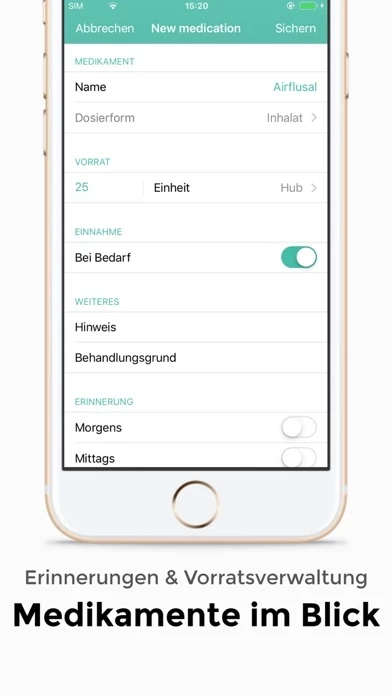
The SaniQ Asthma app supports you with daily challenges: It reliably reminds you to take measurements and take medication.